Occupational Exposure to Hexavalent Chromium, Nickel and PAHs: A Mixtures Risk Assessment Approach Based on Literature Exposure Data from European Countries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search and Data Collection
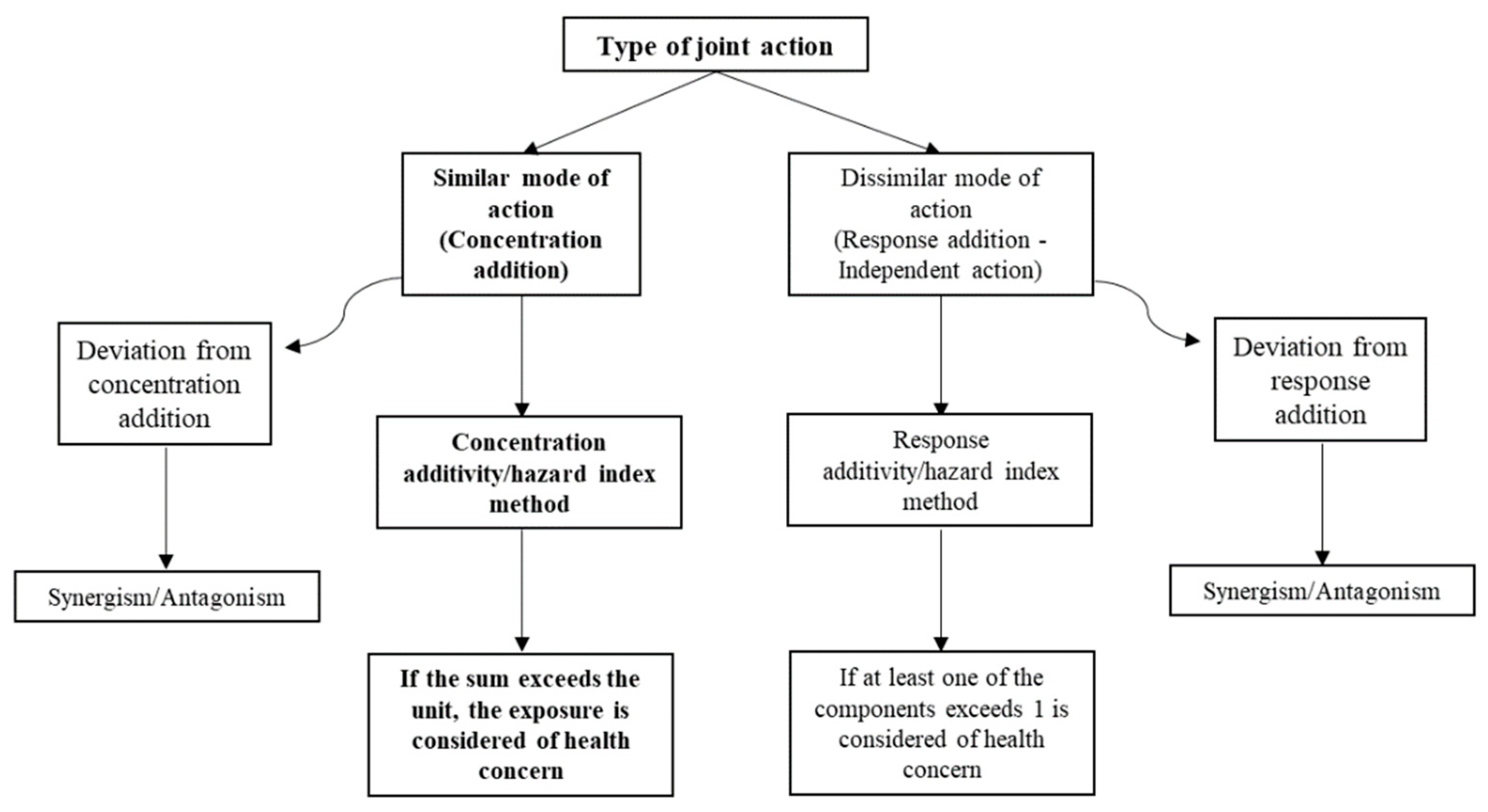
2.2. Determination of the Sum of Risk Quotients
3. Results
3.1. Characteristics of the Studies
3.2. Overall Exposure Biomarkers in Urine
3.3. Risk Quotients in Welding Activities with Exposure to Cr(VI) and Ni
3.4. Risk Quotients after Exposure to Cr(VI), Ni and PAHs
3.5. Effect Biomarkers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Annangi, B.; Bonassi, S.; Marcos, R.; Hernández, A. Biomonitoring of humans exposed to arsenic, chromium, nickel, vanadium, and complex mixtures of metals by using the micronucleus test in lymphocytes. Mutat. Res.-Rev. Mutat. Res. 2016, 770, 140–161. [Google Scholar] [CrossRef] [PubMed]
- CDC/NIOSH. Criteria for a Recommended Standard. Occupational Exposure to Hexavalent Chromium; DHHS (NIOSH) Publication No. 2013–128; Centres for Desease Control and Prevention: Atlanta, GA, USA, 2013.
- IARC. Chromium (VI) Compounds. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2018; pp. 147–167. [Google Scholar]
- IARC. Arsenic, Metals, Fibres and Dusts: Nickel and Nickel Compounds. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; pp. 169–218. [Google Scholar]
- Barchowsky, A.; O’Hara, K.A. Metal-induced cell signaling and gene activation in lung diseases. Free Radic. Biol. Med. 2003, 34, 1130–1135. [Google Scholar] [CrossRef]
- Grimsrud, T.K.; Berge, S.R.; Haldorsen, T.; Andersen, A. Exposure to different forms of nickel and risk of lung cancer. Am. J. Epidemiol. 2002, 156, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, J.P.; Biradar, M.S. Primary concept of nickel toxicity—An overview. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Hartwig, A.; Arand, M.; Epe, B.; Guth, S.; Jahnke, G.; Lampen, A. Mode of Action-Based Risk Assessment of Genotoxic Carcinogens; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1787–1877. ISBN 0123456789. [Google Scholar]
- Šimko, P. Polycyclic aromatic hydrocarbons. Saf. Anal. Foods Anim. Orig. 2016, 3, 441–460. [Google Scholar] [CrossRef]
- Li, G.; Hu, R.; Wang, N.; Yang, T.; Xu, F.; Li, J.; Wu, J.; Huang, Z.; Pan, M.; Lyu, T. Cultivation of microalgae in adjusted wastewater to enhance biofuel production and reduce environmental impact: Pyrolysis performances and life cycle assessment. J. Clean. Prod. 2022, 355, 131768. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Pan, M.; Hao, Y.; Hu, R.; Xiao, W.; Li, G.; Lyu, T. Valorisation of microalgae residues after lipid extraction: Pyrolysis characteristics for biofuel production. Biochem. Eng. J. 2022, 179, 108330. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef]
- European Commission. SCOEL/REC/404 Polycyclic Aromatic Hydrocarbon Mixtures Containing Benzo[a]pyrene (PAH). Recommendation from the Scientific Committee on Occupational Exposure Limits; Publications Office of the European Union: Luxembourg, 2016. [CrossRef]
- Jameson, C.W. Polycyclic aromatic hydrocarbons and associated occupational exposures. In Tumour Site Concordance and Mechanisms of Carcinogenesis; Baan, R., Stewart, B., Straif, K., Eds.; International Agency for Research on Cancer (IARC): Lyon, France, 2019; Chapter 7; pp. 59–64. [Google Scholar]
- IARC. Benzo(a)pyrene. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 423–428. ISBN 9780123864543. [Google Scholar]
- Pesch, B.; Lehnert, M.; Weiss, T.; Kendzia, B.; Menne, E.; Lotz, A.; Heinze, E.; Behrens, T.; Gabriel, S.; Schneider, W.; et al. Exposure to hexavalent chromium in welders: Results of the WELDOX II field study. Ann. Work Expo. Health 2018, 62, 351–361. [Google Scholar] [CrossRef]
- Weiss, T.; Pesch, B.; Lotz, A.; Gutwinski, E.; Van Gelder, R.; Punkenburg, E.; Kendzia, B.; Gawrych, K.; Lehnert, M.; Heinze, E.; et al. Levels and predictors of airborne and internal exposure to chromium and nickel among welders-Results of the WELDOX study. Int. J. Hyg. Environ. Health 2013, 216, 175–183. [Google Scholar] [CrossRef]
- Lißner, L.; Kuhl, T.; Kauppinen, T.; Uuksulainen, S. Exposure to Carcinogens and Work-Related Cancer: A Review of Assessment Methods European Risk Observatory—European Risk Observatory Report; European Agency for Safety and Health at Work; Publications Office of the European Union: Luxembourg, 2014. [CrossRef]
- IARC. Chemical agents and related occupations. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100 Pt F, 9–562. [Google Scholar]
- Wang, T.; Feng, W.; Kuang, D.; Deng, Q.; Zhang, W.; Wang, S.; He, M.; Zhang, X.; Wu, T.; Guo, H. The effects of heavy metals and their interactions with polycyclic aromatic hydrocarbons on the oxidative stress among coke-oven workers. Environ. Res. 2015, 140, 405–413. [Google Scholar] [CrossRef]
- Kortenkamp, A.; Faust, M. Regulate to reduce chemical mixture risk. Science 2018, 361, 224–226. [Google Scholar] [CrossRef] [Green Version]
- Rotter, S.; Beronius, A.; Boobis, A.R.; Hanberg, A.; van Klaveren, J.; Luijten, M.; Machera, K.; Nikolopoulou, D.; van der Voet, H.; Zilliacus, J.; et al. Overview on legislation and scientific approaches for risk assessment of combined exposure to multiple chemicals: The potential EuroMix contribution. Crit. Rev. Toxicol. 2018, 48, 796–814. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Hu, W.; Rom, W.N.; Costa, M.; Tang, M.S. Chromium(VI) exposure enhances polycyclic aromatic hydrocarbon-DNA binding at the p53 gene in human lung cells. Carcinogenesis 2003, 24, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Muthusamy, S.; Xia, Q.; Lal, V.; Denison, M.S.; Ng, J.C. Micronucleus formation by single and mixed heavy metals/loids and PAH compounds in HepG2 cells. Mutagenesis 2015, 30, 593–602. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Martín, F.J.; Fan, Y.; Carreira, V.; Ovesen, J.L.; Vonhandorf, A.; Xia, Y.; Puga, A. Long-term coexposure to hexavalent chromium and B[a]P causes tissue-specific differential biological effects in liver and gastrointestinal tract of mice. Toxicol. Sci. 2015, 146, 52–64. [Google Scholar] [CrossRef] [Green Version]
- Hernández, A.F.; Tsatsakis, A.M. Human exposure to chemical mixtures: Challenges for the integration of toxicology with epidemiology data in risk assessment. Food Chem. Toxicol. 2017, 103, 188–193. [Google Scholar] [CrossRef]
- Drakvik, E.; Altenburger, R.; Aoki, Y.; Backhaus, T.; Bahadori, T.; Barouki, R.; Brack, W.; Cronin, M.T.D.; Demeneix, B.; Hougaard Bennekou, S.; et al. Statement on advancing the assessment of chemical mixtures and their risks for human health and the environment. Environ. Int. 2020, 134, 105267. [Google Scholar] [CrossRef]
- EU. Commission Chemicals Strategy for Sustainability Towards a Toxic-Free Environment. J. Chem. Inf. Model. 2013, 53, 1689–1699. [Google Scholar]
- Kienzler, A.; Bopp, S.K.; van der Linden, S.; Berggren, E.; Worth, A. Regulatory assessment of chemical mixtures: Requirements, current approaches and future perspectives. Regul. Toxicol. Pharmacol. 2016, 80, 321–334. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Committee; More, S.J.; Bampidis, V.; Benford, D.; Bennekou, S.H.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Koutsoumanis, K.; Naegeli, H.; et al. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA J. 2019, 17, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greim, H.; Snyder, R. (Eds.) Toxicology and Risk Assessment; John Wiley & Sons, Ltd.: Chichester, UK, 2008; ISBN 9780470868959. [Google Scholar]
- Lebret, E. Information Needs from Policy Makers Translated in Terms of Functionality of HBM Mixture Information Deliverable Report Deadline: November 2018. 2020. Available online: https://www.hbm4eu.eu/result/deliverables/ (accessed on 14 July 2021).
- Sarigiannis, D.A.; Hansen, U. Considering the cumulative risk of mixtures of chemicals—A challenge for policy makers. Environ. Health A Glob. Access Sci. Source 2012, 11 (Suppl. S1), S18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kortenkamp, A.; Mengelers, M.; Vinggaard, A.M.; Silva, M.J.; Slama, R.; Vermeulen, R.; Vlaanderen, J.; Ottenbros, I.; Viegas, S.; Gomes, B.; et al. Deliverable Report AD 15.1 Plan for Development of Case Studies. 2019. Available online: https://core.ac.uk/download/pdf/196530897.pdf (accessed on 4 January 2021).
- Beronius, A.; Zilliacus, J.; Hanberg, A.; Luijten, M.; van der Voet, H.; van Klaveren, J. Methodology for health risk assessment of combined exposures to multiple chemicals. Food Chem. Toxicol. 2020, 143, 111520. [Google Scholar] [CrossRef]
- European Commission. SCOEL/REC/386 Chromiun VI Compounds. Recommendation from the Scientific Committee on Occupational Exposure Limits; European Commission: Luxembourg, 2017.
- European Chemicals Agency (ECHA). Committee for Risk Assessment—RAC. Opinion on Scientific Evaluation of Occupational Exposure Limits for Nickel and Its Compounds; European Chemicals Agency (ECHA): Helsinki, Finland, 2018.
- European Chemicals Agency (ECHA). RAC—Committee for Risk Assessment. In Note on Reference Dose-Response Relationship for the Carcinogenicity of Pitch, Coal Tar, High Temperature and on PBT and vPvB Properties; European Chemicals Agency (ECHA): Helsinki, Finland, 2018. [Google Scholar]
- Viegas, S.; Jeddi, M.Z.; Hopf, N.B.; Bessems, J.; Palmen, N.; Galea, K.S.; Jones, K.; Kujath, P.; Duca, R.C.; Verhagen, H.; et al. Biomonitoring as an underused exposure assessment tool in occupational safety and health context—Challenges and way forward. Int. J. Environ. Res. Public Health 2020, 17, 5884. [Google Scholar] [CrossRef]
- Santonen, T.; Alimonti, A.; Bocca, B.; Duca, R.C.; Galea, K.S.; Godderis, L.; Göen, T.; Gomes, B.; Hanser, O.; Iavicoli, I.; et al. Setting up a collaborative European human biological monitoring study on occupational exposure to hexavalent chromium. Environ. Res. 2019, 177, 108583. [Google Scholar] [CrossRef]
- Viegas, S.; Martins, C.; Bocca, B.; Bousoumah, R.; Duca, R.C.; Galea, K.S.; Godderis, L.; Iavicoli, I.; Janasik, B.; Jones, K.; et al. HBM4EU Chromates Study: Determinants of Exposure to Hexavalent Chromium in Plating, Welding and Other Occupational Settings. Int. J. Environ. Res. Public Health 2022, 19, 3683. [Google Scholar] [CrossRef]
- Teuschler, L.K.; Hertzberg, R.C. Current and future risk assessment guidelines, policy, and methods development for chemical mixtures. Toxicology 1995, 105, 137–144. [Google Scholar] [CrossRef]
- Ausschuss für Gefahrstoffe (AGS). Technische Regeln für Gefahrstoffe. Risikobezogenes Maßnahmenkonzept für Tätigkeiten mit Krebser—Zeugenden Gefahrstoffen; Committee on Hazardous Substances, 2021; Volume 165. Available online: https://www.baua.de/DE/Angebote/Rechtstexte-und-Technische-Regeln/Regelwerk/TRGS/TRGS-910.html (accessed on 7 April 2021).
- Stridsklev, I.C.; Schaller, K.H.; Langård, S. Monitoring of chromium and nickel in biological fluids of stainless steel welders using the flux-cored-wire (FCW) welding method. Int. Arch. Occup. Environ. Health 2004, 77, 587–591. [Google Scholar] [CrossRef]
- Stridsklev, I.C.; Schaller, K.H.; Langård, S. Monitoring of chromium and nickel in biological fluids of grinders grinding stainless steel. Int. Arch. Occup. Environ. Health 2007, 80, 450–454. [Google Scholar] [CrossRef]
- Gil, F.; Hernández, A.F.; Márquez, C.; Femia, P.; Olmedo, P.; López-Guarnido, O.; Pla, A. Biomonitorization of cadmium, chromium, manganese, nickel and lead in whole blood, urine, axillary hair and saliva in an occupationally exposed population. Sci. Total Environ. 2011, 409, 1172–1180. [Google Scholar] [CrossRef]
- Pesch, B.; Lotz, A.; Koch, H.M.; Marczynski, B.; Casjens, S.; Käfferlein, H.U.; Welge, P.; Lehnert, M.; Heinze, E.; Van Gelder, R.; et al. Oxidatively damaged guanosine in white blood cells and in urine of welders: Associations with exposure to welding fumes and body iron stores. Arch. Toxicol. 2015, 89, 1257–1269. [Google Scholar] [CrossRef] [Green Version]
- Wultsch, G.; Nersesyan, A.; Kundi, M.; Mišík, M.; Setayesh, T.; Waldherr, M.; Vodicka, P.; Vodickova, L.; Knasmüller, S. Genotoxic and Cytotoxic Effects in Exfoliated Buccal and Nasal Cells of Chromium and Cobalt Exposed Electroplaters. J. Toxicol. Environ. Health-Part A Curr. Issues 2017, 80, 651–660. [Google Scholar] [CrossRef]
- Hoffmeyer, F.; Raulf-Heimsoth, M.; Weiss, T.; Lehnert, M.; Gawrych, K.; Kendzia, B.; Harth, V.; Henry, J.; Pesch, B.; Brüning, T. Relation between biomarkers in exhaled breath condensate and internal exposure to metals from gas metal arc welding. J. Breath Res. 2012, 6, 027105. [Google Scholar] [CrossRef]
- Gube, M.; Ebel, J.; Brand, P.; Göen, T.; Holzinger, K.; Reisgen, U.; Kraus, T. Biological effect markers in exhaled breath condensate and biomonitoring in welders: Impact of smoking and protection equipment. Int. Arch. Occup. Environ. Health 2010, 83, 803–811. [Google Scholar] [CrossRef]
- Hoffmeyer, F.; Weiß, T.; Lehnert, M.; Pesch, B.; Berresheim, H.; Henry, J.; Raulf-Heimsoth, M.; Broding, H.C.; Bünger, J.; Harth, V.; et al. Increased metal concentrations in exhaled breath condensate of industrial welders. J. Environ. Monit. 2011, 13, 212–218. [Google Scholar] [CrossRef]
- Hulo, S.; Chérot-Kornobis, N.; Howsam, M.; Crucq, S.; de Broucker, V.; Sobaszek, A.; Edme, J.L. Manganese in exhaled breath condensate: A new marker of exposure to welding fumes. Toxicol. Lett. 2014, 226, 63–69. [Google Scholar] [CrossRef]
- Riccelli, M.G.; Goldoni, M.; Andreoli, R.; Mozzoni, P.; Pinelli, S.; Alinovi, R.; Selis, L.; Mutti, A.; Corradi, M. Biomarkers of exposure to stainless steel tungsten inert gas welding fumes and the effect of exposure on exhaled breath condensate. Toxicol. Lett. 2018, 292, 108–114. [Google Scholar] [CrossRef]
- Persoons, R.; Arnoux, D.; Monssu, T.; Culié, O.; Roche, G.; Duffaud, B.; Chalaye, D.; Maitre, A. Determinants of occupational exposure to metals by gas metal arc welding and risk management measures: A biomonitoring study. Toxicol Lett. 2014, 231, 135–141. [Google Scholar] [CrossRef]
- Lehnert, M.; Weiss, T.; Pesch, B.; Lotz, A.; Zilch-Schöneweis, S.; Heinze, E.; Van Gelder, R.; Hahn, J.U.; Brüning, T. Reduction in welding fume and metal exposure of stainless steel welders: An example from the WELDOX study. Int. Arch. Occup. Environ. Health 2014, 87, 483–492. [Google Scholar] [CrossRef]
- Wegner, R.; Radon, K.; Heinrich-Ramm, R.; Seemann, R.; Riess, A.; Koops, F.; Poschadel, B.; Szadkowski, D. Biomonitoring results and cytogenetic markers among harbour workers with potential exposure to river silt aerosols. Occup. Environ. Med. 2004, 61, 247–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuhmacher, M.; Domingo, J.L.; Agramunt, M.C.; Bocio, A.; Müller, L. Biological monitoring of metals and organic substances in hazardous-waste incineration workers. Int. Arch. Occup. Environ. Health 2002, 75, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Wegner, R.; Heinrich-Ramm, R.; Nowak, D.; Olma, K.; Poschadel, B.; Szadkowski, D. Lung function, biological monitoring, and biological effect monitoring of gemstone cutters exposed to beryls. Occup. Environ. Med. 2000, 57, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agramunt, M.C.; Domingo, A.; Domingo, J.L.; Corbella, J. Monitoring internal exposure to metals and organic substances in workers at a hazardous waste incinerator after 3 years of operation. Toxicol. Lett. 2003, 15, 83–91. [Google Scholar] [CrossRef]
- Mari, M.; Schuhmacher, M.; Domingo, J.L. Levels of metals and organic substances in workers at a hazardous waste incinerator: A follow-up study. Int. Arch. Occup. Environ. Health 2009, 82, 519–528. [Google Scholar] [CrossRef]
- Wultsch, G.; Mišík, M.; Nersesyan, A.; Knasmueller, S. Genotoxic effects of occupational exposure measured in lymphocytes of waste-incinerator workers. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2011, 28, 3–7. [Google Scholar] [CrossRef]
- Kettelarij, J.; Nilsson, S.; Midander, K.; Lidén, C.; Julander, A. Snapshot of cobalt, chromium and nickel exposure in dental technicians. Contact Dermat. 2016, 75, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Beattie, H.; Keen, C.; Coldwell, M.; Tan, E.; Morton, J.; McAlinden, J.; Smith, P. The use of bio-monitoring to assess exposure in the electroplating industry. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Zare Jeddi, M.; Hopf, N.B.; Viegas, S.; Price, A.B.; Paini, A.; van Thriel, C.; Benfenati, E.; Ndaw, S.; Bessems, J.; Behnisch, P.A.; et al. Towards a systematic use of effect biomarkers in population and occupational biomonitoring. Environ. Int. 2021, 146, 106257. [Google Scholar] [CrossRef]
- Cherrie, J.W.; Levy, L. Managing Occupational Exposure to Welding Fume: New Evidence Suggests a More Precautionary Approach is Needed. Ann. Work Expo. Health 2019, 64, 1–4. [Google Scholar] [CrossRef]
- Maître, A.; Collot-Fertey, D.; Anzivino, L.; Marques, M.; Hours, M.; Stoklov, M. Municipal waste incinerators: Air and biological monitoring of workers for exposure to particles, metals, and organic compounds. Occup. Environ. Med. 2003, 60, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colman Lerner, J.E.; Elordi, M.L.; Orte, M.A.; Giuliani, D.; de los Angeles Gutierrez, M.; Sanchez, E.Y.; Sambeth, J.E.; Porta, A.A. Exposure and risk analysis to particulate matter, metals, and polycyclic aromatic hydrocarbon at different workplaces in Argentina. Environ. Sci. Pollut. Res. 2018, 25, 8487–8496. [Google Scholar] [CrossRef] [PubMed]
- Campo, L.; Hanchi, M.; Sucato, S.; Consonni, D.; Polledri, E.; Olgiati, L.; Saidane-Mosbahi, D.; Fustinoni, S. Biological Monitoring of Occupational Exposure to Metals in Electric Steel Foundry Workers and Its Contribution to 8-Oxo-7,8-Dihydro-2′-Deoxyguanosine Levels. Int. J. Environ. Res. Public Health 2020, 17, 1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, H.; Fang, B.; Hao, K.; Wang, H.; Zhang, L.; Wang, M.; Hao, Y.; Wang, X.; Wang, Q.; Yang, W.; et al. Combined effects of exposure to polycyclic aromatic hydrocarbons and metals on oxidative stress among healthy adults in Caofeidian, China. Ecotoxicol. Environ. Saf. 2022, 230, 113168. [Google Scholar] [CrossRef] [PubMed]
- Olea, N.; Fernández, M.F.; Mustieles, V.; David, A.; Barouki, R.; Fini, J.; Demeneix, B.; Lambrechts, N.; Schoeters, G.; Vingaard, A.M.; et al. Criteria for Prioritization of Biomarkers of Effect Deliverable Report WP 14 Effect Biomarkers. 2017. Available online: https://www.hbm4eu.eu/wp-content/uploads/2017/03/HBM4EU_D14.1_Criteria-Prioritization-Biomarkers-of-Effect.pdf (accessed on 12 January 2021).
- Sudha, S.; Prathyumnan, S.; Keyan, K.S.; Joseph, S.; Vasudevan, B.S.G.; Sasikala, K. Evaluation of DNA damage induction and repair inhibition in welders exposed to hexavalent chromium. Asian Pac. J. Cancer Prev. 2010, 11, 95–100. [Google Scholar]
- Balachandar, V.; Arun, M.; Mohana Devi, S.; Velmurugan, P.; Manikantan, P.; Karthick Kumar, A.; Sasikala, K.; Venkatesan, C. Evaluation of the genetic alterations in direct and indirect exposures of hexavalent chromium [Cr(VI)] in leather tanning industry workers North Arcot District, South India. Int. Arch. Occup. Environ. Health 2010, 83, 791–801. [Google Scholar] [CrossRef] [PubMed]
- El Safty, A.M.K.; Samir, A.M.; Mekkawy, M.K.; Fouad, M.M. Genotoxic Effects Due to Exposure to Chromium and Nickel Among Electroplating Workers. Int. J. Toxicol. 2018, 37, 234–240. [Google Scholar] [CrossRef] [Green Version]
- Galea, K.S.; Porras, S.P.; Viegas, S.; Bocca, B.; Bousoumah, R.; Duca, R.C.; Godderis, L.; Iavicoli, I.; Janasik, B.; Jones, K.; et al. HBM4EU chromates study—Reflection and lessons learnt from designing and undertaking a collaborative European biomonitoring study on occupational exposure to hexavalent chromium. Int. J. Hyg. Environ. Health 2021, 234, 113725. [Google Scholar] [CrossRef]
- Louro, H.; Heinälä, M.; Bessems, J.; Buekers, J.; Vermeire, T.; Woutersen, M.; van Engelen, J.; Borges, T.; Rousselle, C.; Ougier, E.; et al. Human biomonitoring in health risk assessment in Europe: Current practices and recommendations for the future. Int. J. Hyg. Environ. Health 2019, 222, 727–737. [Google Scholar] [CrossRef]
- Ermler, S.; Scholze, M.; Kortenkamp, A. Genotoxic mixtures and dissimilar action: Concepts for prediction and assessment. Arch. Toxicol. 2014, 88, 799–814. [Google Scholar] [CrossRef] [Green Version]


| Substances | Tolerable Levels in Air (µg/m3) a | Limit Values in Urine | |
|---|---|---|---|
| µg/g Creatinine | µg/L | ||
| Chromium (VI) | 1.0 | 1.20 c | 1.63 d |
| Nickel b | 6.0 | 2.21 d | 3.00 e |
| Benzo(a)pyrene | 0.07 | 1.03 d (for 1-OHP) | 1.40 f (for 1-OHP) |
| Occupational Setting | Activities | Biomarkers of Exposure in Urine (µg/g Creatinine) | Ref. | ||
|---|---|---|---|---|---|
| U-Cr | U-Ni | U-1-OHP | |||
| Incinerator operations, boiler and furnace maintenance, control panel, and waste-gas-washing | 0.39 ± 0.24 a | 3.7 ± 1.9 a | <0.04 ± 0.3 a | [57] | |
| 0.26 a | 3.03 a | 0.1 a | [59] | ||
| Waste incineration | 4.8 ± 3.2 (<4; 11.0) a | 0.20 a | [60] | ||
| 0.50 ± 0.58 (exposure 1–3 months) 1.89 ± 4.59 (exposure 3–8 months) 0.26 ± 0.24 a (exposure 8–11 months) | 9.18 ± 7.47 (exposure 1–3 months) 12.12 ± 8.31 (exposure 3–8 months) 7.69 ± 5.57 a (exposure 8–11 months) | [61] | |||
| Manufacture of railway vehicles | Welding e | 0.72 (IQR 0.58–1.20) | 1.56 (IQR 1.01–2.48) | [52] | |
| Welding industries | 0.32 (IQR 0.97) (pre-shift) 0.54 (IQR 1.39) (post-shift) | 1.11 (IQR 1.6) (pre-shift) 1.47 (IQR 2.27) (post-shift) | [50] | ||
| 2.3 (0.7–7.3) b | 1.5 (0.5–3.2) b | [55] | |||
| Flux cored arc welding | 3.8 (0.48–18.0) (first void) 3.2 (<0.24–30.1) (before work) 3.96 (0.34–40.7) (after work) a | 1.9 (1.05–5.59) (first void) 2.7 (1.11–4.37) (before work) 2.5 (0.56–5.0) (after work) a | [44] | ||
| Flux cored and gas metal arc welding | <1.35 (IQR <0.74–<3.24) | <2.56 (IQR <1.37–<4.39) | [17] | ||
| Gas metal arc welding | 0.39 | 1.57 | [54] | ||
| Tungsten inert gas welding | 0.71 (0.36–1.27) (before Friday shift) 0.74 (0.41–1.21) (after Friday shift) 0.59 (0.26–1.00) (after weekend break) | 0.76 (0.37–1.40) (before Friday shift) 1.11 (0.59–0.79) (after Friday shift) 0.83 (0.31–1.38) (after weekend break) | [53] | ||
| Shipyards and other industries | 0.85 (IQR 0.53–1.32) (pre-shift) 0.90 (IQR 0.56–1.55) (post-shift) | 1.53 (IQR 1.05–3.37) (pre-shift) 1.67 (IQR 0.89–2.97) (post-shift) | [16] | ||
| Gas metal arc welders with massive or flux cored wire of stainless steel; Flux-cored arc welding of mild steel; Tungsten inert gas welding | 0.88 d | 2.07 d | [47] | ||
| Stainless steel grinding | 1.6 (<0.15–4.6) (first void) 1.40 (<0.14–4.5) (before work) 1.40 (<0.13–5.5) (post shift) | 3.79 (0.68–10.6) (first void) 3.39 (0.25–11.1) (before work) 4.56 (<0.53–11.5) (post shift) | [45] | ||
| Harbour | Harbour dredgers and lighters | 0.22 d | 0.7 (0.1–7.3) | [56] | |
| Iron and steel industry | Production, polishing and shaving of stainless-steel vessels and other metallurgical processes | 0.42 (0.10–19.65) | 0.25 (0.12–51.01) | [46] | |
| Dental laboratory | Preparation of prostheses and of metal constructions for dental crowns | 0.14 c,d | 4.35 c,d | [62] | |
| Electroplating companies | Processes of electroplating, preparatory work, maintenance activities, polishing of electroplated items, and ancillary tasks | 1.10 d 1.47 (electroplating workers) d | 4.26 d 4.88 (electroplating workers) d | [63] | |
| Workshops specializing in the processing of beryls | Gemstone cutting | ≤4 h/week: 1.18 (pre-shift); 0.85 (post-shift) d >4 h/week: 0.81 (pre-shift); 0.66 (post-shift) d | [58] | ||
| Welding Activity | Cr | Ni | SRQ | Ref. | ||
|---|---|---|---|---|---|---|
| Exposure Level (µg/m3) a | RQ | Exposure Level (µg/m3) | RQ | |||
| Welding f | 20.0 b,c,e | 20.0 | 70.0 c,e | 11.7 | 31.7 | [52] |
| 6.30 b,g,h | 6.30 | 2.80 g,h | 0.47 | 6.77 | [55] | |
| Flux cored arc welding | 11.3 i | 11.3 | 50.4 a,i | 8.40 | 19.7 | [44] |
| 1.80 b,c,d | 1.80 | 2.15 c,d | 0.36 | 2.16 | [17] | |
| Gas metal arc welding | 7.80 b,c | 7.80 | 11.0 c | 1.83 | 9.63 | [17] |
| 0.24 h | 0.24 | 2.70 h | 0.45 | 0.69 | [16] | |
| Shielded metal arc welding | 0.04 h | 0.04 | 0.49 h | 0.082 | 0.12 | [16] |
| 17.0 b,c | 17.0 | 4.00 c | 0.67 | 17.7 | [17] | |
| Tungsten inert gas welding | 10.5 b,c | 10.5 | 2.55 c,d | 0.43 | 10.9 | [17] |
| 0.23 h | 0.23 | 0.67 h | 0.11 | 0.34 | [16] | |
| 1.00 d,i | 1.00 | 1.00 i | 0.17 | 1.17 | [53] | |
| 1.35 b,d,i | 1.35 | 0.75 d,i | 0.13 | 1.48 | [47] | |
| Gas metal arc welding with massive or flux cored wire of stainless steel (high exposure group) | 239.0 b,i | 239.0 | 82.5 i | 13.8 | 252.8 | [47] |
| Flux-cored arc welding of mild steel | 0.60 b,d,i | 0.60 | 0.85 d,i | 0.14 | 0.74 | [47] |
| Welding Activity | U-Cr | U-Ni | SRQ | Ref. | ||
|---|---|---|---|---|---|---|
| Exposure Level | RQ | Exposure Level | RQ | |||
| Welding d | 0.72 µg/g creat | 0.60 | 1.56 µg/g creat e | 0.71 | 1.31 | [52] |
| 2.30 µg/g creat b | 1.92 | 3.10 µg/L b | 1.03 | 2.95 | [55] | |
| 0.54 µg/g creat (post-shift) | 0.45 | 1.47 µg/g creat e (post-shift) | 0.67 | 1.12 | [50] | |
| Flux cored arc welding | 3.96 µg/g creat (after work) a | 3.30 | 2.50 µg/g creat (after work) a,e | 1.13 | 4.43 | [44] |
| 0.43 µg/g creat c | 0.36 | 2.83 µg/L | 0.94 | 1.30 | [17] | |
| Gas metal arc welding | 0.83 µg/g creat c | 0.69 | 3.76 µg/L c | 1.25 | 1.94 | [17] |
| 0.39 µg/g creat | 0.33 | 1.57 µg/g creat e | 0.71 | 1.04 | [54] | |
| 0.94 µg/g creat (post-shift) | 0.78 | 2.87 µg/L (post-shift) | 0.96 | 1.74 | [16] | |
| Shielded metal arc welding | 0.74 µg/g creat (post-shift) | 0.62 | 1.20 µg/L (post-shift) | 0.40 | 1.02 | [16] |
| 1.17 µg/g creat c | 0.98 | 1.98 µg/L | 0.66 | 1.64 | [17] | |
| Tungsten inert gas welding | 0.61 µg/g creat c | 0.51 | 0.75 µg/L c | 0.25 | 0.76 | [17] |
| 0.78 µg/g creat (post-shift) | 0.65 | 1.31 µg/L (post-shift) | 0.44 | 1.09 | [16] | |
| 0.74 µg/g creat (after Friday shift) | 0.62 | 1.11 µg/g creat e (after Friday shift) | 0.50 | 1.12 | [53] | |
| 0.50 µg/L c,e | 0.31 | 0.75 µg/L c | 0.25 | 0.56 | [47] | |
| Gas metal arc welders with massive or flux cored wire of stainless steel (high exposure group) | 13.53 µg/L e | 8.29 | 7.92 µg/L | 2.64 | 10.9 | [47] |
| Flux-cored arc welding of mild steel | 0.50 µg/L c | 0.31 | 2.70 µg/L | 0.90 | 1.21 | [47] |
| Activities | U-Cr | U-Ni | U-1-OHP | SRQ | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Exposure Level (µg/g Creat) | RQ | Exposure Level (µg/g Creat) | RQ | Exposure Level (µg/g Creat) | RQ | |||
| Incinerator operations, boiler and furnace maintenance, control panel, and waste-gas-washing | 0.39 | 0.33 | 3.70 b | 1.68 | 0.30 b | 0.29 | 2.30 | [57] |
| 0.26 | 0.22 | 3.03 b | 1.37 | 0.10 b | 0.10 | 1.69 | [59] | |
| Laboratory activities | 0.57 | 0.48 | 2.30 b | 1.04 | 0.20 b | 0.19 | 1.71 | [57] |
| 0.19 | 0.16 | 2.55 b | 1.16 | 0.02 a,b | 0.019 | 1.34 | [59] | |
| Administrative activities | 0.22 | 0.18 | 3.60 b | 1.63 | 0.02 a,b | 0.019 | 1.83 | [57] |
| 0.26 | 0.22 | 2.34 b | 1.06 | 0.02 a,b | 0.019 | 1.30 | [59] | |
| Activities [Ref.] | Exposure Biomarkers | Effect Biomarkers | Overall Outcomes | SRQ | |
|---|---|---|---|---|---|
| Harbour dredgers and lighters [56] | U-Cr (0.22 µg/g creat) and U-Ni (0.7 µg/g creat) | MN | Significantly higher frequency in controls vs. exposed | Controls: 14.2 MNs/1000 cells; Exposed: 11.6 MNs/1000 cells | 0.36 |
| SCE | No significant differences between exposed and controls | Controls: 10.2 SCE rate; Exposed: 10.0 SCE rate | |||
| Incinerator operations, boiler and furnace maintenance, control panel, and waste-gas-washing [61] | U-Cr (short-0.5 ± 0.58; medium-1.89 ± 4.59; long-exposure-0.26 ± 0.24 µg/g creat) U-Ni (short-9.18 ± 7.47; medium- 12.12 ± 8.31; long-exposure-7.69 ± 5.7 µg/g creat) | MN | No significant differences between exposed and controls | Controls: 1.3/1000 cells Short-exposure group: 1.6/1000 cells, Medium-exposure group: 1.7/1000 cells Long-exposure group: 1.7/1000 cells | 2.10 (long-exposure) |
| Comet assay | Short exposure group: 6.5 tail factor; Medium exposure group: 6.3 tail factor; Long exposure group: 6.7 tail factor Controls: 7.1 tail factor | ||||
| Gemstone cutting [58] | U-Ni ≤4 h/week: 1.18 µg/g creat (pre-shift); 0.85 (post-shift) >4 h/week: 0.81 (pre-shift); 0.66 (post-shift) | SCE | No significant differences between exposed groups | Short exposure group: 9.91 SCE/cell; Long exposure group: 9.70 SCE/ cell | - |
| Tungsten inert gas welding [53] | U-Cr 0.71 µg/g creat (0.36–1.27) (T1) 0.74 µg/g creat (0.41–1.21) T2) 0.59 µg/g creat (0.26–1.00) (T3) U-Ni 0.76 µg/g creat (0.37–1.40) (T1) 1.11 µg/g creat (0.59–0.79) (T2) 0.83 µg/g creat (0.31–1.38) (T3) | MDA-EBC | Significant decrease at T2 | 2.79 nM, 2.98 nM and 2.43 nM at T0, T1 and T2, respectively | 1.12 |
| HNE-EBC | Significant increase at T0 than at T2 | 0.53 nM, 0.48 nM and 0.51 nM at T0, T1 and T2, respectively | |||
| H2O2-EBC | Significant increase at T1 than at T0 and T2 | 0.18, 0.25, and 0.16 μM at T0, T1 and T2, respectively | |||
| Welding [50] | U-Cr (0.32 µg/g creat (IQR 0.97) (pre-shift) 0.54 µg/g creat (IQR 1.39) (post-shift) U-Ni 1.11 µg/g creat (IQR 1.6) (pre-shift) 1.47 µg/g creat (IQR 2.27) (post-shift) | H2O2-EBC | Significant increase in welders vs. controls | 56 ± 19 pM/µg vs. 6.6 ± 2.2 pM/µg | 1.12 |
| Nitrate/tyrosine ratio | 0.71 ± 0.13 nM/µg vs. 0.22 ± 0.04 nM/µg | ||||
| Gas metal arc welders with massive or flux cored wire of stainless steel; Flux-cored arc welding of mild steel; Tungsten inert gas welding in shipyards industries [47] | U-Cr (0.88 µg/g creat) U-Ni (2.07 µg/g creat) | Urinary 8-oxoGuo | No significant differences between welding techniques (adjusted for creatinine) | High-exposure group: 6.59 µg/g creat; Flux-cored arc welding of mild steel: 7.62 µg/g creat; Tungsten inert gas welders: 6.41 µg/g creat | 0.56 for TIGW |
| Urinary 8-oxodGuo | High-exposure group: 4.28 µg/g creat; Flux-cored arc welding of mild steel: 3.79 µg/g creat; Tungsten inert gas welders: 4.41 µg/g creat | 10.9 for high exposure group a | |||
| 8-oxodGuo/106dGuo in white blood cells | High-exposure group: 5.53/106dGuo; Flux-cored arc welding of mild steel: 4.79/106dGuo; Tungsten inert gas welders: 1.98/106dGuo | 1.21 for FCAW of mild steel | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, A.M.; Viegas, S.; Louro, H.; Göen, T.; Santonen, T.; Luijten, M.; Kortenkamp, A.; Silva, M.J. Occupational Exposure to Hexavalent Chromium, Nickel and PAHs: A Mixtures Risk Assessment Approach Based on Literature Exposure Data from European Countries. Toxics 2022, 10, 431. https://doi.org/10.3390/toxics10080431
Tavares AM, Viegas S, Louro H, Göen T, Santonen T, Luijten M, Kortenkamp A, Silva MJ. Occupational Exposure to Hexavalent Chromium, Nickel and PAHs: A Mixtures Risk Assessment Approach Based on Literature Exposure Data from European Countries. Toxics. 2022; 10(8):431. https://doi.org/10.3390/toxics10080431
Chicago/Turabian StyleTavares, Ana Maria, Susana Viegas, Henriqueta Louro, Thomas Göen, Tiina Santonen, Mirjam Luijten, Andreas Kortenkamp, and Maria João Silva. 2022. "Occupational Exposure to Hexavalent Chromium, Nickel and PAHs: A Mixtures Risk Assessment Approach Based on Literature Exposure Data from European Countries" Toxics 10, no. 8: 431. https://doi.org/10.3390/toxics10080431
APA StyleTavares, A. M., Viegas, S., Louro, H., Göen, T., Santonen, T., Luijten, M., Kortenkamp, A., & Silva, M. J. (2022). Occupational Exposure to Hexavalent Chromium, Nickel and PAHs: A Mixtures Risk Assessment Approach Based on Literature Exposure Data from European Countries. Toxics, 10(8), 431. https://doi.org/10.3390/toxics10080431










